JOIN OUR WHATSAPP GROUP. CLICK HERE
Matter and Materials Grade 10 Notes
Matter and Materials Grade 10 Notes On this page, we will examine Matter and Materials for students in Grades 8, 9, 10, and 11:
Different materials can be used to create various objects (the matter from which objects are made). A cupboard, for example, is made of wood, nails, hinges, and knobs (the components). The materials’ qualities will influence the object’s properties. In the case of the cupboard, the strength of the wood and metals make the cabinet strong and long-lasting. Understanding the qualities of materials is critical so that we can use them in our homes, industries, and other applications.
What is Matter?
Matter is anything that has weight and takes up space. Everything you can see and touch is made up of matter. Matter exists in three main forms: solids, liquids, and gases. It also has properties that we can describe through density, solubility, conductivity, magnetism, etc.
Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that live around us. Even our own human bodies are made of matter!
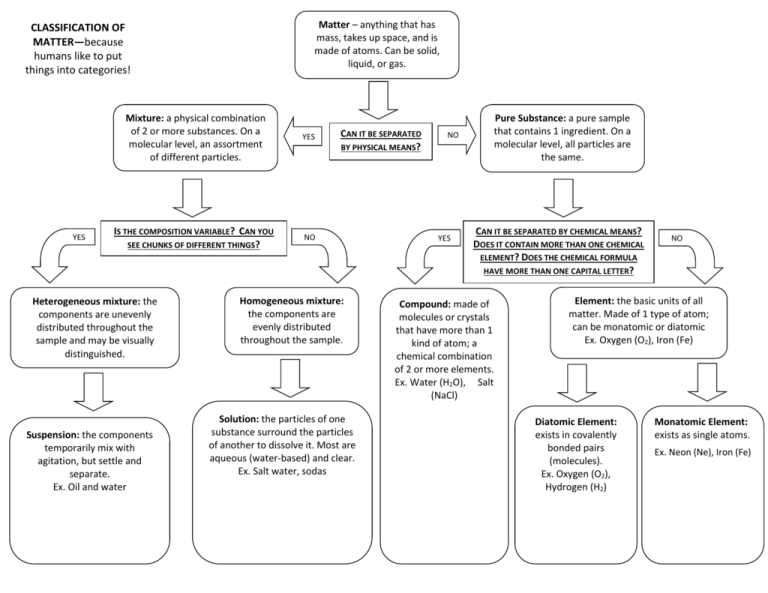
Classification of matter with examples
Key Points
- Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
- A chemical substance is composed of one type of atom or molecule.
- A mixture is composed of different types of atoms or molecules that are not chemically bonded.
- A heterogeneous mixture is a mixture of two or more chemical substances where the various components can be visually distinguished.
- A homogeneous mixture is a type of mixture in which the composition is uniform and every part of the solution has the same properties.
- Various separation techniques exist in order to separate matter, including include distillation, filtration, evaporation and chromatography. Matter can be in the same phase or in two different phases for this separation to take place.
Key Terms
- mixture: Something that consists of diverse, non-bonded elements or molecules.
- element: A chemical substance that is made up of a particular kind of atom and cannot be broken down or transformed by a chemical reaction.
- substance: A form of matter that has constant chemical composition and characteristic properties. It is composed of one type of atom or molecule.
Quiz Video
Resources
https://www.generationgenius.com/properties-of-matter-for-kids/
https://studylib.net/doc/6631784/classification-of-matter
JOIN OUR TELEGRAM CHANNEL. CLICK HERE




Be the first to comment